Product Description
Helicobacter pylori (H.pylori) IgG/ IgM Test Kit (Colloidal Gold Method) is used for the in vitro qualitative detection of Helicobacter pylori antibody IgG/IgA in human serum/plasma/whole blood samples. For people who have not been treated for Helicobacter pylori eradication, combined with clinical and other laboratory indicators, it is used for the auxiliary diagnosis of Helicobacter pylori infection.Antibody detection products cannot be used for the recent judgment of the evaluation of the eradication effect of Helicobacter pylori.
【Reagents And Materials Supplied】
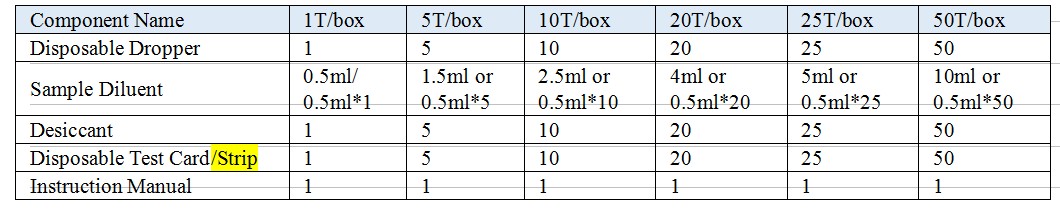
Model: Test Card, Test Strip
【SHELF LIFE AND STORAGE】
Store in a dry place at 2~30 ℃ away from light.
2.Transport at 2-37℃ for 20 days
3. After opening the inner packaging, the test card will become invalid due to moisture absorption, please use it within 1 hour.
4. The shelf life of the test kit is 12 months from date of manufacture.
【Specimen Collection】
1. Serum (S): Collect whole blood into a collection tube (does not contain anticoagulants such as heparin, EDTA and sodium citrate) by venipuncture, let it stand for 30 minutes for blood coagulation, and then centrifuge the blood to obtain the supernatant Serum specimen of liquid.
2. Plasma (P): Collect whole blood in a collection tube (containing anticoagulants, such as heparin, EDTA, and sodium citrate) by venipuncture, and then centrifuge the blood to obtain a plasma sample.
3. Whole blood (WB): Collect whole blood through a blood sampling device. WB can be transferred directly to the test card by pipetting.
【Test procedure】
Before opening the bag, please leave it at room temperature. Take the test device out of the sealed bag and use it as soon as possible. The best results will be obtained if the measurement is performed within one hour.
2.Dispense 35 µL of serum/plasma or whole blood into the sample wells of the test card.
3.Dispense 1 drop of buffer directly from the buffer bottle, or use a calibrated pipette to transfer 40µL of buffer to the sample well.
4.The result should be between 10 and 20 minutes, but not more than 30 minutes
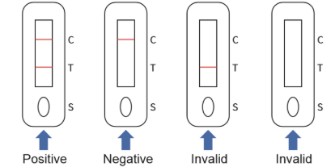
【INTERPRETATION OF ASSAY RESULT】
1.NEGATIVE RESULT:
If only the C line develops, the test indicates that no detectable hepatitis C virus is present in the specimen. The result is negative or non-reactive.
2. POSITIVE RESULT:
In addition to the presence of the C line, if the T line develops, the test indicates the presence of hepatitis C virus. The result is hepatitis C virus positive or reactive.
3. INVALID
If the C line does not develop, the assay is invalid regardless of color development of the T line as indicated below. Repeat the assay with a new device.
Online Message
Products Recommended
Please give us a message